usp class vi compliant
Class VI is the most stringent and requires. Chapters 87 and 88 describe procedures for testing and evaluation polymeric materials and medical devices.
Specially formulated for long term sealing.

. Both ISO 10993 and USP Class VI define testing requirements for biocompatibility the ability of a material to perform a desired function without causing adverse effects on the human body. Testing is commonly done as per USP which requires three types of. USP Class VI and FDA White List Silicone and Organic Elastomer Compounds for Healthcare Products.
Most applications are fairly benign to elastomers. Dursan can be applied to more durable base materials like stainless steel to. USP Class VI testing helps ensure that all materials used in industrial processes are biocompatible with any substances or chemicals they may encounter.
AFT Fluorotec can manufacture a wide range of components using our USP Class VI PTFE compliant material and are supplying. The result is a non-toxic bio-inert surface that is USP Class VI compliant that wont contaminate or otherwise harm processes or people. Master Bond systems are very versatile and can be used for both disposable and.
United States Pharmacopeia USP 26 NF21 2003 Class VI. Class testing is needed when manufacturing drugs to identify its low toxicity compliance while ensuring it meets all biocompatibility standards. Certified USP Class VI Silicones.
By ensuring that a material is non-toxic and wont result in immunological rejection biocompatibility testing ensures that a rubber is safe for use with. USP Class VI Medical Packaging Requirements. Certificates of Analysis COAs report the test results for a specific batch of materials.
USP Class VI materials EPDM Silicone Fluorocarbon and Perfluoroelastomer 24 materials which are compliant to FDA 21 CF R1772600. All these special grade products have passed this rigorous test. I - VI with USP Class VI being the strictest requiring that the material exhibit very low.
Sterile and diaphragm valves have USP Class VI PTFE material in them and sanitary pumps require Class VI O-Rings and sealing material. USP Class VI Compliant Materials for Medical and Pharmaceutical Products Meets USP Class VI requirements for use in medical and pharmaceutical applications. USP Class VI testing is conducted by producing an extract of the product with different extraction fluids such as polyethylene glycol and vegetable oil and injecting it in specimen rabbits and mice in vivo alive to observe the biological response to the extract.
Three chapters are applicable to elastomers plastics and polymeric materials. Compounds made without animal-derived ingredients BSETSE. Coatings like Dursan prevent system cross contamination while improving corrosion resistance and durability.
Specialty Silicone Products SSP provides complete certifications to demonstrate the quality of its SSP-2390 Series USP Class VI FDA and RoHS compliant silicones. Many plastics manufacturers find it advantageous to have their materials classified especially if their plastic resins are a likely candidate to be used in medical devices. Therefore it is.
Pharmacopeia a private non-government organization that promotes the public health by establishing state-of-the-art standards to ensure the quality of medicines and other health care technologies. Typical applications for our FDA NSF 51 USDA materials are disposable medical devices surgical instruments and medical fluid dispensing components as well as a wide variety of food and beverage. Excelon RNT 68 Food Beverage Tubing.
Testing was performed by Pacific BioLabs on September 16 2015 in compliance with the standards published in the USP Biocompatibility Testing standards USP. PPE manufactures medical and pharmaceutical grade gaskets and seals including O-rings sanitary gaskets and other high performance seals from a range of 15 USP Class VI compliant elastomers-. Excelon RNT 1065 Vinyl Tubing.
Has a full range of specialty adhesives epoxies primers for polyolefins UV curables and silicones that have been fully tested to meet USP Class VI requirements. Primarily biopharmaceutical manufacturers use USP Class VI for their process equipment. Table 1 shows our standard programme FDA compliant com- FDA and USP class VI compliant.
The United States Pharmacopoeia USP 30 NF 25 2007 standard also known as Class VI is widely used to comply with stringent FDA regulations for products that come in contact with the human body. The focus of these tests is determining the biological reactivity of the material. An article of commerce that is recognized in the USPNF complies with USPNF standards when it meets all of the requirements stated in the articles monograph applicable General Chapters and the General Notices with monograph requirements superseding those of the General Chapters and General Notices in any cases where requirements differ.
For plastics they have six different classes based on duration and application. USP Class VI materials meet the most stringent requirements and include silicones that pass a systemic toxicity test an intracutaneous test and an implantation test. The USP defines six plastics classes from class I to class VI with class VI being the most rigorous and most frequently requested certification.
USP Class VI Approved Plastic Materials. 24 materials which are compliant to FDA 21 CFR1772600. USP stands for US.
Specially formulated for long term sealing. Sil 714001 USP class VI Silicone 1 70 Yes transl. In order to mass produce a device intended for humans to wear on a constant basis like a blood sugar monitor.
44 0 1909 560 203. Graco Company have been tested for compliance to USP Class VI 70C plastic. Yet some suppliers that use compliant ingredients may still not be able to guarantee a compliant end-product.
C-Flex ULTRA biopharma pump tubing. AdvantaFlex TPE Biopharma Tubing. The USP outlines classes for plastic materials ie.
Moulded O-rings class 1 less than 10 furnace black These can be produced in all possible dimensions up to diameter 1400 mm internal. SIMONA PP-H USP Class VI sheet is ideal for applications requiring biocompatibility testing standards defined by ISO 109931. For most patient-contact applications a material that meets US Pharmacopeia USP Class VI andor ISO 109933 will be required.
The USP Class VI compounds must be made from ingredients with clear histories of biocompatibility that meet tighter requirements for leachates. Tests of the provided material samples passed all requirements and have been approved for. Sil 714002 USP class VI Silicone 1 70 Yes transl.
Excelon RNT 60 PVC Vinyl Lab Tubing. FEP Shrink Tubing 131 161. Certificates of Conformance COC attest to a batchs.
Darcoid and Parker offer a wide range of USP Class VI and FDA.

Usp Class Vi Gaskets Seals Usp Class 6 O Rings Ppe

Standard Fda And Usp Class Vi Compliant Materials For All Types Of Hygienic Connections Repassa
Dursan Passes Usp Class Vi Testing Why Is That Important
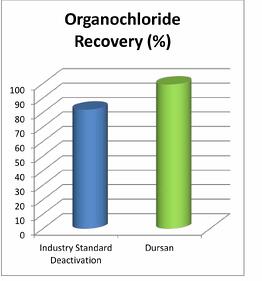
Dursan Passes Usp Class Vi Testing Why Is That Important

Usp Class Vi Zulassung Was Bedeutet Das Reichelt Chemietechnik Magazin

What Is Usp Class Vi Testing Tbl Plastics

Dursan Passes Usp Class Vi Testing Why Is That Important

Fda Usda Nsf51 Usp Class Vi Compliant Seals Products

Usp Class Vi Zulassung Was Bedeutet Das Reichelt Chemietechnik Magazin

Why You Need Certified Usp Class Vi Silicones Specialty Silicone Products Inc

Usp Class Vi Foster Corporation
Usp Class Vi Zulassung Was Bedeutet Das Reichelt Chemietechnik Magazin

Iso 10993 Vs Usp Class Vi Medical Molding And Bicompatible Rubber The Rubber Group

Bal Seal Engineering Achieves Usp Class Vi And Iso 10993 5 Compliance For Medical Sealing Polymers Bal Seal Engineering
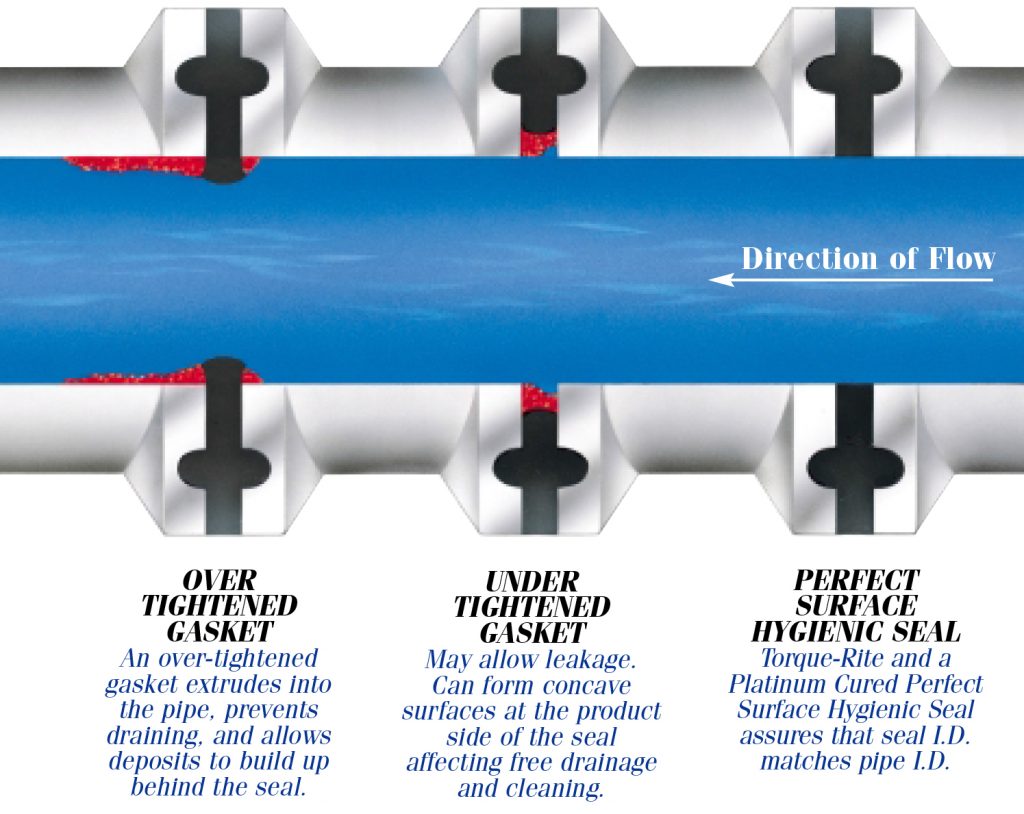
Standard Fda And Usp Class Vi Compliant Materials For All Types Of Hygienic Connections Repassa




